|
The
content of the labeling provided with drug listing (e.g., professional labeling
(package insert), consumer labeling, carton labels and container labels) is
included in the same SPL file as the other drug listing information. Carton and
container labels include the content of the Drug Facts or equivalent for animal
drug products and the content of the Principal Display Panel including the image
of the entire label as a single jpg file.
 | Product Label | Product Label, Word Document Setup and Structured Data such as Indications, Contraindications, Conditions of Use, Drug Interactions etc. |
 | Word Document | Product Label - Word Document Processing - Setup |
 | Indications | Indication information including maximum dose, conditions of use and contraindications if any |
 | Adverse Reactions | Known adverse reactions if any |
 | Drug Interactions | Known drug interactions if any |
 | Document Sections | SPL Product Label Document Sections as imported from Word Document |
 | Word Document | Product Label - Word Document Processing - Setup |
 | Effective Time | Date of the Document - YYYYDDMM Date Format - 20090101 - SPL requires each label section having an effective date. This date will be used when importing. |
 | File | Browse for Word Document containing the Label Information |
 | Image Target Folder | Target folder for images being extracted from Word Document if any. Note: SPL File must be placed in same folder as the images. |
 | Extract
Images | Yes
= Extracts Images from Word Document and saves them to file, existing files
are overwritten. No = Images are not exctracted from Word Document but still
linked in SPL - Image files must be placed manually in the Image Target
Folder. |
 | Load
Document Title | Yes
= Loads the text under HIGHLIHTS OF PRESCRIBING INFORMATION to General Data
- Title, No = Leaves the General Data - Title unchanged. |
 | Boxed
Warning Title | The
title of the Boxed Warning as defined in Word Document, needed to match the
correct FDA SPL document section |
 | Section Style | Identifies the sections in the Product Label Word document. This setting represents the entry point for
FDA SPL Software for SPL R4 from which text, images and tables are converted from Word to SPL format. |
 | Sub Section Style | Identifies the subsection style in the Word document. Subsections can dynamically be added to the Product Label Document |
 | Excerpt Style | Specifies the style for the highlights (excerpts) of the SPL. Text is loaded from the word document and converted into correct SPL structure. |
 | Section
Numbering From Word | If
this option is set, the section and sub section numbering will be added as
defined in Word Document |
 | Check Characters | Allows to define a list of characters which occurrence is checked while processing the Word Document. If a specified character is found the conversion is paused and the word document paragraph containing the character is highlighted. |
 | Ignore Word Table Cell Borders | When this option is set
FDA SPL Software for SPL R4 converts tables as proposed by FDA. if this option is not set the table border setting from the Word Document is applied |
 | Process Cross References | If this option is set
FDA SPL Software for SPL R4 processes cross references in the Word Document and converts them to links in the resulting SPL Document. Formatting in Word Document: Each Cross Reference must be included within brackets [] - e.g. [1] Thus a Cross Reference List has to be formatted as follows: [1], [2], [3] etc.
It is strongly recommended
to Use Word Hyperlinks to Section and Subsection Headers instead of the
automatic Word Fields.
|
 | Postprocess Product Label | If this option is set, the application
post processes the Product Label and adds Indications, Contraindications, Conditions of use and other structured label data to the SPL file |
|
Product
Label - Word
Document Content and Format
|
|
According to the SPL
specification a correct Structured Product Label must contain following
information:
|
|
Highlights/Excerpts
|
|
BOXED WARNING
[Excerpt Style]
Highlight Text
INDICATIONS AND USAGE [Excerpt Style]
Highlight Text
DOSAGE AND ADMINISTRATION [Excerpt Style]
Highlight Text
DOSAGE FORMS AND STRENGTHS [Excerpt Style]
Highlight Text
CONTRAINDICATIONS [Excerpt Style]
Highlight Text
WARNINGS AND PRECAUTIONS [Excerpt Style]
Highlight Text
ADVERSE REACTIONS [Excerpt Style]
Highlight Text
DRUG INTERACTIONS [Excerpt Style]
Highlight Text
USE IN SPECIFIC POPULATIONS [Excerpt Style]
Highlight Text
|
DRUG ABUSE AND DEPENDENCE
[Excerpt Style]
Highlight Text
OVERDOSAGE [Excerpt Style]
Highlight Text
DESCRIPTION [Excerpt Style]
Highlight Text
CLINICAL PHARMACOLOGY [Excerpt Style]
Highlight Text
NONCLINICAL TOXICOLOGY [Excerpt Style]
Highlight Text
CLINICAL STUDIES
[Excerpt Style]
Highlight Text
REFERENCES
[Excerpt Style]
Highlight Text
HOW SUPPLIED/STORAGE AND HANDLING [Excerpt Style]
Highlight Text
PATIENT COUNSELING INFORMATION [Excerpt Style]
Highlight Text
|
|
Product Label Document Content
|
|
WARNING
1 INDICATIONS AND USAGE [Section Style]
1.1 [text] [Subsection Style]
1.2 [text] [Subsection Style]
1.3 [text] [Subsection Style]
2 DOSAGE AND ADMINISTRATION [Section Style]
2.1 [text] [Subsection Style]
2.2 [text] [Subsection Style]
3 DOSAGE FORMS AND STRENGTHS [Section Style]
3.1 [text] [Subsection Style]
3.2 [text] [Subsection Style]
4 CONTRAINDICATIONS [Section Style]
4.1 [text] [Subsection Style]
4.2 [text] [Subsection Style]
5 WARNINGS AND PRECAUTIONS [Section Style]
5.1 [text] [Subsection Style]
5.2 [text] [Subsection Style]
5.3 [text] [Subsection Style]
6 ADVERSE REACTIONS [Section Style]
6.1 [text] [Subsection Style]
6.2 [text] [Subsection Style]
7 DRUG INTERACTIONS [Section Style]
7.1 [text] [Subsection Style]
7.2 [text] [Subsection Style]
8 USE IN SPECIFIC POPULATIONS [Section Style]
8.1 Pregnancy [Subsection Style]
8.2 Labor and Delivery [Subsection Style]
8.3 Nursing Mothers [Subsection Style]
8.4 Pediatric Use [Subsection Style]
8.5 Geriatric Use [Subsection Style]
|
9 DRUG ABUSE AND DEPENDENCE
[Section Style]
9.1 Controlled Substance [Subsection Style]
9.2 Abuse [Subsection Style]
9.3 Dependence [Subsection Style]
10 OVERDOSAGE [Section Style]
11 DESCRIPTION [Section Style]
12 CLINICAL PHARMACOLOGY [Section Style]
12.1 Mechanism of Action [Subsection Style]
12.2 Pharmacodynamics [Subsection Style]
12.3 Pharmacokinetics [Subsection Style]
13 NONCLINICAL TOXICOLOGY [Section Style]
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
[Subsection Style]
13.2 Animal Toxicology and/or Pharmacology
[Subsection Style]
14 CLINICAL STUDIES [Section Style]
14.1 [text] [Subsection Style]
14.2 [text] [Subsection Style]
14.3 [text] [Subsection Style]
15 REFERENCES [Section Style]
16 HOW SUPPLIED/STORAGE AND HANDLING [Section Style]
16.1 [text] [Subsection Style]
16.2 [text] [Subsection Style]
17 PATIENT COUNSELING INFORMATION [Section Style]
17.1 [text] [Subsection Style]
17.2 [text] [Subsection Style]
17.3 [text] [Subsection Style]
|
|
Complete List of possible allowed Document
Sections in alphabetical order
|
-
ABUSE
-
ADVERSE REACTIONS
-
ANIMAL PHARMACOLOGY AND OR TOXICOLOGY
-
BOXED WARNING
-
CARCINOGENESIS AND MUTAGENESIS AND IMPAIRMENT OF FERTILITY
-
CLINICAL PHARMACOLOGY
-
CLINICAL STUDIES
-
CONTRAINDICATIONS
-
CONTROLLED SUBSTANCE
-
DEPENDENCE
-
DESCRIPTION
-
DOSAGE AND ADMINISTRATION
-
DOSAGE FORMS AND STRENGTHS
-
DRUG AND OR LABORATORY TEST INTERACTIONS
-
DRUG ABUSE AND DEPENDENCE
-
DRUG INTERACTIONS
-
ENVIRONMENTAL WARNING
-
FOOD SAFETY WARNING
-
GENERAL PRECAUTIONS
-
GERIATRIC USE
-
GUARANTEED ANALYSIS OF FEED
-
HOW SUPPLIED
-
INACTIVE INGREDIENT
-
INDICATIONS AND USAGE
-
INFORMATION FOR OWNERS/CAREGIVERS
-
INFORMATION FOR PATIENTS
-
LABOR AND DELIVERY
-
LABORATORY TESTS
-
MECHANISM OF ACTION
-
MICROBIOLOGY
-
NONCLINICAL TOXICOLOGY
-
NONTERATOGENIC EFFECTS
-
NURSING MOTHERS
-
OVERDOSAGE
-
OTC - ACTIVE INGREDIENT
-
OTC - ASK DOCTOR
-
OTC - ASK DOCTOR/PHARMACIST
-
OTC - DO NOT USE
-
OTC - KEEP OUT OF REACH OF CHILDREN
-
OTC - PREGNANCY OR BREAST FEEDING
-
OTC - PURPOSE
-
OTC - QUESTIONS
-
OTC - STOP USE
-
OTC - WHEN USING
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
PEDIATRIC USE
-
PHARMACODYNAMICS
-
PHARMACOKINETICS
-
PRECAUTIONS
-
PREGNANCY
-
RECENT MAJOR CHANGES
-
REFERENCES
-
RESIDUE WARNING
-
SAFE HANDLING WARNING
-
SPL INDEXING DATA ELEMENTS
-
SPL LISTING DATA ELEMENTS
-
SPL MEDGUIDE
-
SPL PATIENT PACKAGE INSERT
-
SPL UNCLASSIFIED
-
STORAGE AND HANDLING
-
TERATOGENIC EFFECTS
-
USE IN SPECIFIC POPULATIONS
-
USER SAFETY WARNINGS
-
VETERINARY INDICATIONS
-
WARNINGS AND PRECAUTIONS
-
WARNINGS
|
|
Rules
to be observed in Product Label - MS Word document for correct SPL conversion |
|
Correct spelled
Section and Excerpt Titles
Correct Titles according to the SPL HL7 definition have to be used - the
spelling of the titles must be identical to the specification - see also the
document structure above.
Correct Paragraph
Styles
Paragraph styles are the entry points in excerpts /highlights, sections
and subsections. The paragraph styles in the Word document to be converted
must be correctly set, else FDA SPL Software for SPL R4 is not able to convert text, images and
tables. Text contained in Excerpts, Sections and Subsections can have any
other style assigned.
Correct Paragraph
Formatting
FDA SPL Software for SPL R4 can only convert correctly formatted data. Images and Tables must be
placed in their own paragraphs. Inline Images and/or tables cannot not be
converted.
Cross References
FDA SPL Software for SPL R4 can build links inside the SPL XML document being transformed. For
this purpose the cross references must be correctly named in the Word
Document.
Example: In section "Warning and Precautions" a cross reference to
section "Use in specific populations" subsection "Pediatric Use"
has to be added.
The naming of the cross reference must be "Pediatric Use".
Clean Document
Because Microsoft Word tracks internally all changes ever made to a document,
it may happens that the MS Word Document Object Model returns wrong data,
tables, images and text when accessed programmatically as it happens with FDA SPL Software
for SPL R4.
To avoid problems of this type, please do the following once authoring of the
product label is terminated:
-
Open your Word
Document
-
Press Ctrl + A
(Select All)
-
Press Ctrl + C
(Copy to Windows Clipboard)
-
Create a new Word
Document
-
Press Ctrl + V
(Paste previous copied Word Document)
-
Use the new Word
Document created to be processed by FDA SPL Software for SPL R4.
|
|
To Load the Product
Label from Word Document to SPL Document configure the Word Document Setup as
described above, then click on the menu "SPL R4 Document" >
"Import Product Label".
An SPL XML
definition template that contains all document sections defined with identical
spelled titles, is stored in the application installation folder.
The conversion occurs according to this template file in 2 steps:
1) Conversion of the Word document to SPL
2) Assignment of LOINC codes to Subsections using the SPLCodeSystems.mdb
Database in MS Access format provided.
|
|
1) Conversion of
the Word document to SPL
|
|
After the Word
document has been prepared as described in the previous section the conversion
can take place.
Product and Company Name and other header Information are entered in the SPL conversion user interface.
The Word paragraph styles for Excerpt -, section and subsection titles are
specified.
The Word document to be converted and the output folder for SPL are selected.
When conversion is started, FDA SPL Software for SPL R4 copies all files needed for correct SPL
processing and display, to the output folder specified by the user.
A subfolder named SPL will be created. Existing Files will be overwritten.
The Application loads the SPL XML template
contained in the application folder - the Word document to be converted will
be matched against this SPL XML template file.
The sections in the template file are populated by the data contained in the
Word document as follows
FDA SPL Software for SPL R4 reads the Word document paragraph by paragraph. If the paragraph
style in the Word document matches the defined style in FDA SPL Software
for SPL R4 then the
content will be processed as follows:
The paragraph
style in Word matches the [Excerpt
Style]
FDA SPL Software
for SPL R4
looks in
the SPL template file if the corresponding section can be found.
If the section
is found
Any text which style is different from [Excerpt
Style], [Section Style] and [Subsection
Style] will be loaded to the corresponding SPL section as
excerpt.
The operation is logged:
Processing Excerpts - SECTION TITLE
If not found
The excerpt cannot be added to the SPL. It's up to the user to cancel
further processing to correct the error.
The error is logged:
Processing Excerpts - SECTION
TITLE
Error Document Section >SECTION
TITLE< not
found in SPL template
The paragraph
style in Word matches the [Section
Style]
FDA SPL Software
for SPL R4
looks in
the SPL template file if the corresponding section can be found.
If found
Paragraphs following the section title are processed until a new section
is found or the end of document is reached.
Text, images and tables in the section are converted to SPL format
The operation is logged:
Processing SECTION TITLE
If not found
Subsection cannot be added to SPL for that section. It's up to the user
to cancel further processing to correct the error.
The error is logged:
Processing SECTION
TITLE
Error Document Section >SECTION
TITLE< not
found in SPL template
The paragraph
style in Word matches the [Subsection
Style]
FDA SPL Software
for SPL R4
looks in
the SPL template file if the parent section of the subsection can be found.
If found
Paragraphs following the subsection title are processed until a new
subsection or section is found or the end of document is reached.
Text, images and tables in the subsection are converted to SPL format.
The operation is logged:
Processing SECTION TITLE
Processing SUBSECTION
TITLE
If not found
Subsection text, images and tables cannot be added to SPL for that
subsection. It's up to the user to cancel further processing to correct the
error.
The error is logged:
Processing SECTION
TITLE
Error Document
Section >SECTION
TITLE< not
found in SPL template
Text, Table, Cross
Reference and
Image processing
FDA SPL Software for SPL R4
processes the text contained
in excerpts, sections and subsections. Tables are processed in sections and
subsections - the operation is logged.
Images, if any, will be extracted from the Word Document sections and
subsections and saved in the output folder - the operation is, again, logged.
Cross references processing, if requested, is also logged.
Note: The SPL
file is saved also if the user cancels the conversion the resulting
file might be incomplete but can be useful for error resolution.
Note: The appearance of the SPL in the browser is not controlled by FDA SPL Software
for SPL R4
but from the XML style sheets provided by HL7/FDA.
Furthermore there can be slightly differences in the appearance if displayed
in different web browsers.
|
|
LOINC
Code Selection
If FDA SPL Software
for SPL R4
is unable to get an exact LOINC match from a subsection title in the
MS Word Document, then the user is prompted to select the LOINC code by
himself.
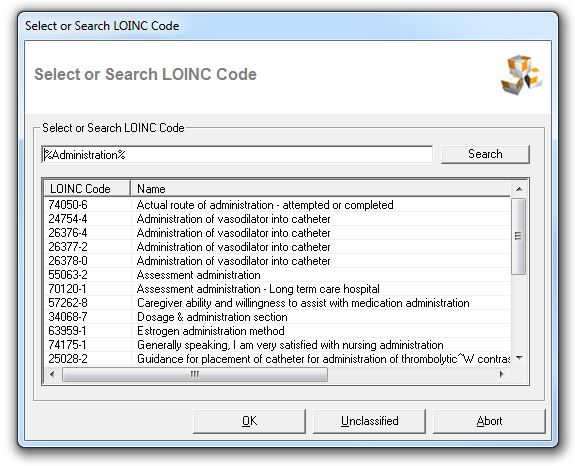
Multiple LOINC matches are found
If the desired LOINC code and text is displayed, then a double click on the
item in the list, will set that LOINC code. The application proceeds with
next document subsection.
No LOINC matches are found
The user can search the LOINC database and
select the LOINC code and name corresponding to the Word document subsection.
Meanings of the command buttons
OK: Apply selected LOINC code
Unclassified: Set
LOINC code for subsection to unclassified
Abort: Abort LOINC code
loading
|
|
During conversion FDA SPL Software for SPL R4 displays an exhaustive log where processing
information, warnings and errors, if any, are displayed.
On following log entries user intervention is needed.
Document
Processing
Error:
Document Section title/Excerpt title spelling has to be checked in word
Document. Spelling must match exactly the specification.
Warning:
A warning during processing. Exact message is
shown in the processing log.
LOINC Code
Processing
Warning:
Multiple LOINC codes are found for the Subsection Title. The user will be
prompted for input.
Error: No LOINC code is found for the
Subsection Title. The user will be prompted for input.
Fatal
Error: Fatal Application Error - e.g. Output Folder does not exist,
or user has not enough permissions to access it. MS Word is not installed etc.
The user should try to correct the error according to the error message. If
the problem persists, the software vendor should be contacted.
Validation
against FDA SPL Software Schema
The application validates the XML SPL file generated against the
FDA SPL Software Schema:
Information Validating XML File against SPL Schema
Important: If the application reports that the SPL file is not valid
according to the FDA SPL Software Schema, FDA might reject the SPL if submitted. Always
correct validation Errors (e.g. Non numeric values in numeric quantity
nominator or denominator fields in ingredients) before submitting your Product
Registration to FDA!
Mismatches after
conversion
Subsections are
missing in converted SPL file
The spelling of the section titles in the Word document have to be checked
against the SPL HL7 document specification.
Images and/or
tables are not correctly placed in the converted SPL file
Tables and images cannot be placed inline to a paragraph - they must
be placed in their own paragraphs. The Word Document has to be checked.
|
 | Indications | Indication
information including maximum dose, conditions of use and contraindications
if any |
 | Indication | |
 | Indication | |
 | Reason Type Code | Indication Reason: prophylaxis, diagnosis or treatment |
 | NCI Code | Indication code from NCI National Cancer Institute Thesaurus - Use
FDA SPL Software Search Function |
 | NCI Display Name | Indication display name NCI National Cancer Institute Thesaurus - Use
FDA SPL Software Search Function |
 | Maximum Dose | |
 | Contraindications | |
 | Conditions Of Use | |
 | Monitoring | |
 | Maximum Dose | |
 | Numerator Value | Value of the maximum dose if any - Number
e.g.. 1,2,3 etc. |
 | Numerator Unit | Unit of measure for value deriving from UCUM - Unified Codes Unit of Measure |
 | Denominator Value | Number
e.g.. 1,2,3 etc. |
 | Denominator Unit | UCUM Time unit |
 | Contraindication | |
 | NCI Code | Contraindication code for each indication deriving from NCI National Cancer Institute Thesaurus - Use
FDA SPL Software Search Function |
 | NCI Display Name | Contraindication name for each indication deriving from NCI National Cancer Institute Thesaurus - Use
FDA SPL Software Search Function |
 | Patient Age | |
 | From | Start Age in Years - Number
e.g.. 1,10,20,30 etc. |
 | To | End Age in Years - Number
e.g.. 1,10,20,30 etc. |
 | Patient Race | |
 | NCI Code | Race Code from NCI National Cancer Institute Thesaurus - Select from List or Use
FDA SPL Software Search Function |
 | NCI Display Name | Race Name from NCI National Cancer Institute Thesaurus - Select from List or Use
FDA SPL Software Search Function |
 | Patient Gender | |
 | NCI Code | Gender - male or female - code from NCI National Cancer Institute Thesaurus - Select from List or Use
FDA SPL Software Search Function |
 | NCI Display Name | Gender - male or female - name from NCI National Cancer Institute Thesaurus - Select from List or Use
FDA SPL Software Search Function |
 | Co Administration | |
 | Substance UNII Code | Co Administration Substance from UNII -Unique Ingredient Identifiers - Use
FDA SPL Software Search Function or see FDA online resources |
 | Substance UNII Display Name | Co Administration Substance from UNII -Unique Ingredient Identifiers - Use
FDA SPL Software Search Function or see FDA online resources |
 | Parameter | |
 | LOINC Code | Parameter monitoring e.g. blood pressure - information from NCI National Cancer Institute Thesaurus - Select from List or Use
FDA SPL Software Search Function |
 | LOINC Display Name | Parameter monitoring e.g. blood pressure - information from NCI National Cancer Institute Thesaurus - Select from List or Use
FDA SPL Software Search Function |
 | Adverse Reaction | |
 | NCI Code | Adverse reaction code from NCI National Cancer Institute Thesaurus - Select from List or Use
FDA SPL Software Search Function |
 | NCI Display Name | Adverse reaction name from NCI National Cancer Institute Thesaurus - Select from List or Use
FDA SPL Software Search Function |
 | Interaction | Interaction Description |
 | Substance UNII Code | Interacting Substance - information from UNII -Unique Ingredient Identifiers - Use
FDA SPL Software Search Function or see FDA online resources |
 | Substance UNII Display Name | Interacting Substance - information from UNII -Unique Ingredient Identifiers - Use
FDA SPL Software Search Function or see FDA online resources |
 | Risks | Drug interaction risks |
 | Risk | |
 | Type Code | NCI concept code for type of drug interaction |
 | Type Display Name | NCI concept code display name for type of drug interaction |
 | Consequence Code | Consequence of the
interaction - NCI National Cancer Institute Thesaurus - Select from List or Use
FDA SPL Software Search Function |
 | Consequence Display Name | Consequence of the
interaction - NCI National Cancer Institute Thesaurus - Select from List or Use
FDA SPL Software Search Function |
 | Document Sections | SPL
Product Label Document Sections as imported from Word Document |
 | Section | Section containing text and images imported
from Word Document.
To add Section Numbering to
the Title, Right Click Product Label > Document Sections - the select
"Add Section Numbering to Product Label".
You can add a custom
Separator between section number and Title.
E.g. Separator= )
Section Title will be Number) Title
|
 | Section | Section
containing text and images imported from Word Document |
 | Section Title | The section title defined must match the Word Document Headings and the headings defined for SPL Documents |
 | Effective Time | Time for the Document Section - is copied from the word document setting upon import - YYYYDDMM Date Format - 20090101 |
 | Section ID | Document Section ID |
 | Section Name | SPL R4 Document Section LOINC name which is loaded when product label is imported from word - text formatted as defined in Word Document Setup Section Style - see FDA online resources for details or select from list |
 | Section Code | SPL R4 Document Section LOINC code which is loaded when product label is imported from word - text formatted as defined in Word Document Setup Section Style - see FDA online resources for details or select from list |
 | Excerpt | Excerpt text loaded from word document and formatted with style defined in Word Document Setup - Excerpt Style |
 | Label Text | |
(c)
sartori-software.com 2018
|
