The QC Scheduler is an Intra- or
Internet based application, that allows worldwide
recording, planning, managing and reporting of Pharmaceutical Product
Filling and Packaging Lots/ Batches for Quality Control purposes.
Products throughput intervals can be defined to survey the production
pipeline in order to fulfill your company's Quality Control needs.
The software aids Sales Managers to
forecast the readiness of Product Lots for delivery.
Different Security Levels allow different
access to the data stored.
An Import Software allows to gather data
periodically from freely configurable 3rd party systems e.g. LIMS
(Laboratory Information Management System), SAP, Oracle etc.
The application functionality can be summarized as follows
- Define Production Locations and
Products
- Access enable Users to the QC
Scheduler Application
- Record, manage an report Production
and Packaging Lots QC - Status
- Document and Report Product Lot
deliveries worldwide
Application
Overview
With QC Scheduler following data
can be easily managed, maintained and reported
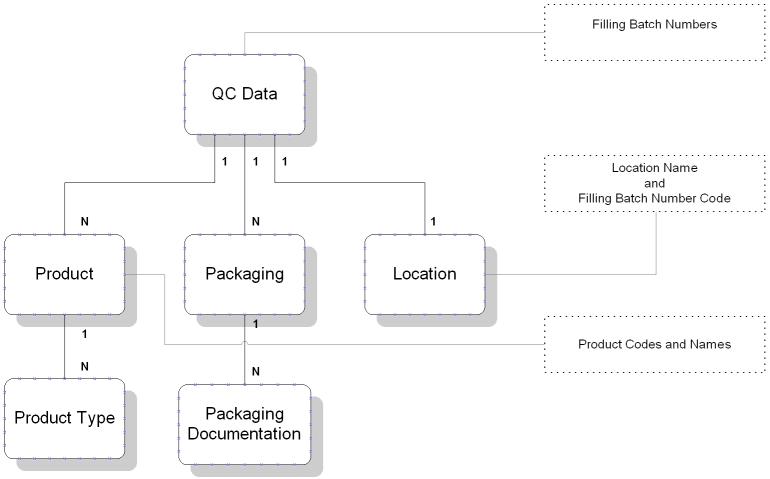
QC
Scheduler
Data Model
System
Requirements
Webserver: Apache, IIS or similar
Database Server: MS SQL Server 2000 or later
Client: Any modern Webbrowser such as
Firefox, Internet Explorer etc.
|