| Introduction |
The Registration Database is an Intra- or
Internet based application that allows worldwide recording, planning, managing and reporting Pharmaceutical Product
Registrations in accordance to Drug Regulatory Affair needs:
-
Define
Master Data such as Manufacturers,
Products, SPC,
Strengths, Packaging Materials, Storage Conditions and Shelf Lives.
-
Keep track
of your Product Registrations by country, store defined product
characteristics such as Ingredients, Filling Sizes and Package Sizes.
-
Link
Product Registrations to MA-Holder information, MOP, PIL, SPC, Packaging
and other documents.
-
Plan, submit and document Product
Registration requests to authorities .
-
Plan, submit and document Variations
of Product Registrations.
-
Document and Report Product
Registration Status worldwide.
-
Easily
Create XEVPRM Product Messages for electronic submission to European
Medicines Agency in accordance with
Article 57(2), second
subparagraph of Regulation (EC) No 726/2004.
Application
Overview



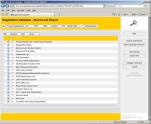
With Registration Database following data
can be easily managed and maintained:
Registration
Database Model
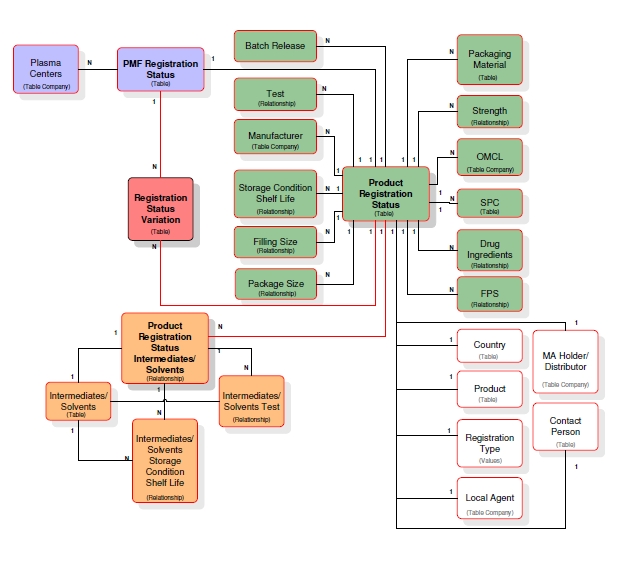
|
| Master
Data |
|
Products
|
Root
Data for Products: Code, Name, Filling Size, Package Size, Strength,
Country, Blood Group, Master MOP, link to file System etc.
|



|
|
Intermediates
|
Intermediates
Master Table: A Product is build by Intermediates e.g. Plasma, Basic
Substances manufactured by third parties
|


 |
|
Companies/
Suppliers
|
Root
Company Data: Name, Address, Contact Data: Manufacturers, MA-Holders
and Suppliers delivering Materials (e.g. Plasma Centres, Basic
Component Manufacturers etc) Laboratories
|


 |
|
Packaging
Materials and Medical Devices
|
Label, Carton, Leaflet, Syringe etc.
|


 |
|
Tests
|
Holds
root data of possible Tests including approved Test Kits to be made
on a Product and/or Intermediate e.g. Nucleic Acid Tests (NAT)
|


 |
|
Bags
|
Holds
Root Data for Blood Bags
|


 |
|
SPC
|
Summary
of Product Characteristics
|


 |
|
Epidemiological
Data
|
Holds
Epidemiological Statistical Data for Donors/Donations in Plasma
Centres over several years and several Viruses
|


 |
|
Countries
|
Country
Root Data: Data can be loaded from this Table when needed
|


 |
|
PSUR
|
Periodic
Safety Update Report
|


 |
| Search,
Report and automated Info Mailing |
|
Search
& Report
|
Custom
Search Functionality (Plugins) to amplify the Application
Functionality based on Company needs
|


 |
|
Email
Settings
|
Table
defining on which Email Addresses change Information has to be sent.
To be configurable by System Owner
|


 |
| User
Management & Security |
|
Application
Users
|
Personnel
Data: Personal Information, Contact Information and Application
Access Permission
|


 |
|
Access
Control and Data Access Rules
|
Access
Control Data defines the Security Matrix for Data access by Persons
|


 |
| Audit
Trail and Access Logging |
|
AuditTrail
|
Audit
Trail of Changes in the Registration Database
|

 |
|
Search
Log
|
Log
Table for Usage of Search And Report Functions
|

 |
(c) sartori-software.com
2012
|